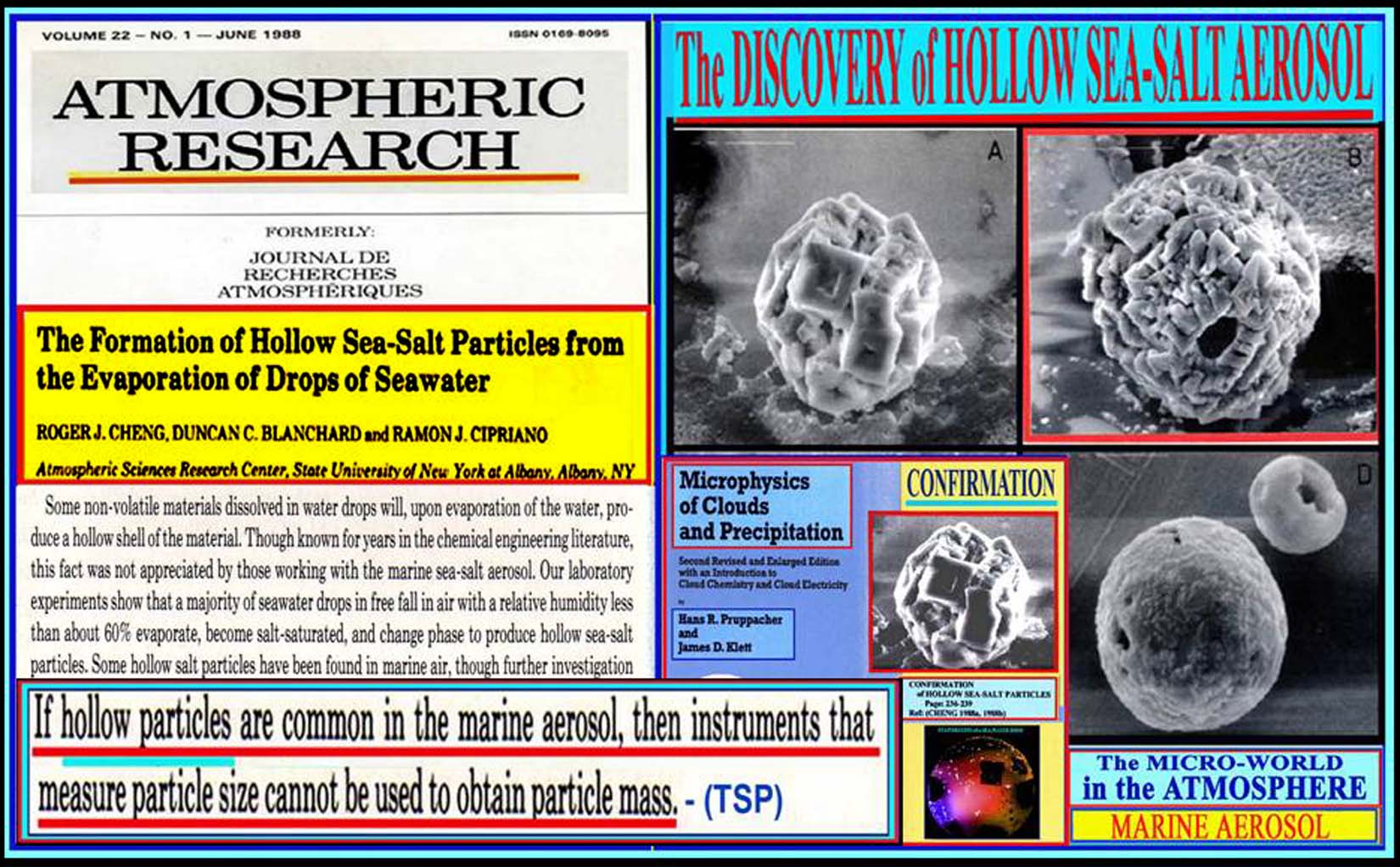
|
The
generation of maritime cloud condensation nuclei (CCN) through
the ejection of jet and film droplets from bursting whitecap-produced
bubbles on the ocean surface has been well documented. The
processes involved in the transformation (evaporation and
crystallization) of these liquid droplets into their solid
form under varying conditions, however, has not previously
attracted much attention from atmospheric researchers.
A
set of laboratory investigations and field observations of
the characteristics, both physical and chemical, of seawater
droplets during phase change in a controlled environment have
revealed the following startling and very significant phenomena:
|
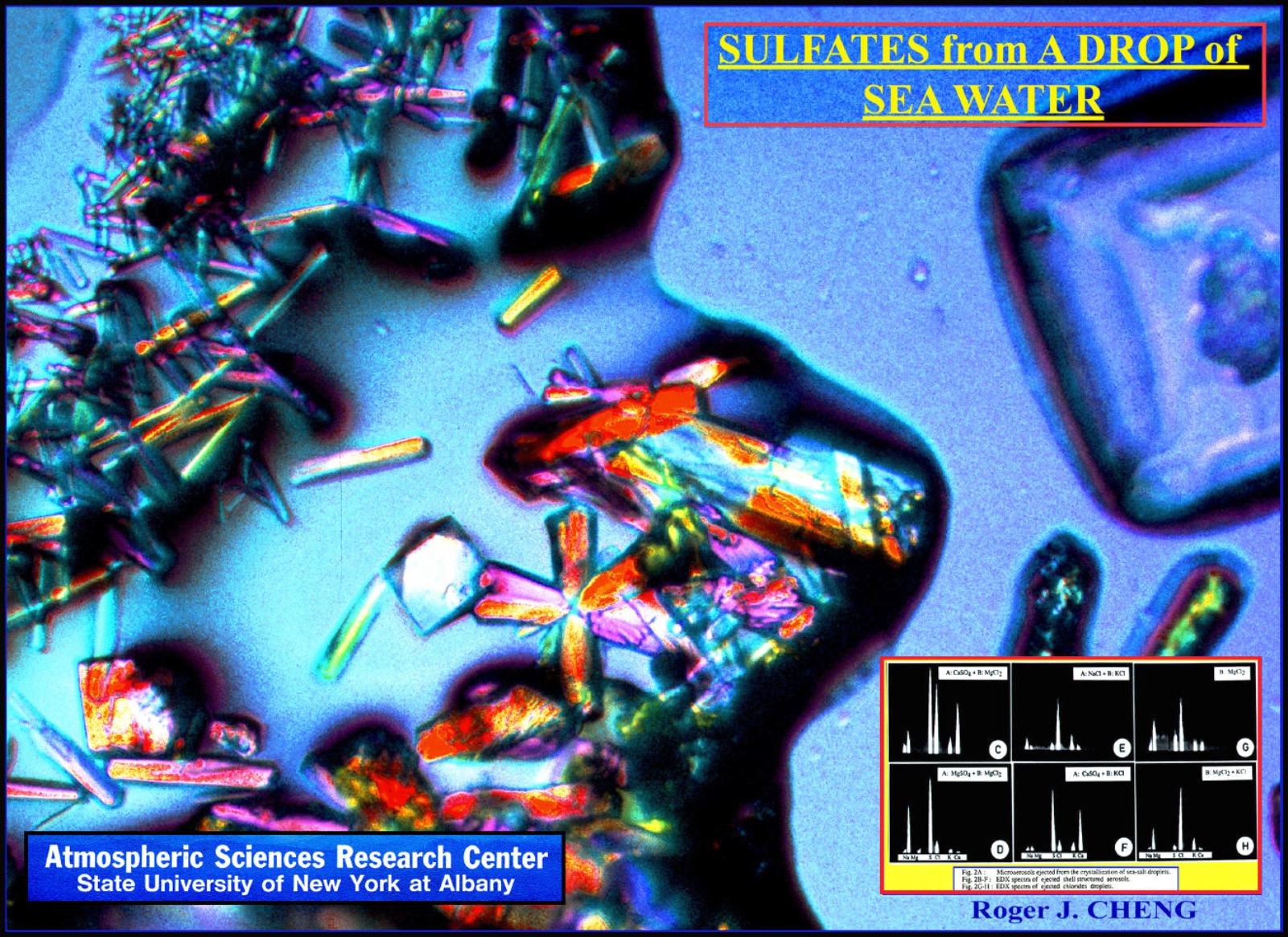
(1)
The ejection of sulfate aerosols (CaSO/4,MgSO/4) with
size range of 0.1mm to 10 mm. The concentration and
chemical composition of the ejected aerosols, identified
by the techniques of scanning electron microscopy and
energy dispersive x-ray spectroscopy, were dependent
on the rate of droplet evaporation. Sea-salt aerosols
could be classified into three categories: (A) NaCI
crystals, (B) Crystals of sulfates (CaSO/4,MgSO/4) and
(C) Chloride droplets (MgCI/2, KCI).
|
|
(2)
The formation of hollow spherical sea-salt particles
(>5 mm). A secondary ejection of aerosols was detected
during melting by the busting of air bubbles, which
were formed when the hollow particle was moved into
a high-moisture environment or dissolved into a water
droplet.
|
| (3)
A thin
film of chlorides (MgCl/2, KCI) observed on the surface
of sea salt particles present a highly hygroscopic surface
to initiate the condensation of water vapor in an environment
with RH As low as 40%. Sodium Chloride (NaCI-75%RH)
plays only a minor role for the formation of cloud droplets
in the marine atmosphere. |
Characterization
of the ejected sulfate aerosols in comparison with field observation,
chemical processes inside the evaporating seawater droplets
and the mechanisms for the generation of secondary aerosols
in the marine atmosphere are presented with illustrations.
.jpg)
|

.jpg)








.jpg)


.jpg)